3 methods for decaffeinating coffee

Discover the fascinating world of decaffeinated coffee, where you’ll learn how caffeine is removed while preserving the aromas and flavors. This article also addresses health concerns and the environmental impact associated with these methods. Dive into the history and composition of decaffeinated coffee to better understand this caffeine-free alternative, while familiarizing yourself with its subtleties.
When you savor a cup of decaffeinated coffee, have you ever wondered how caffeine is extracted from coffee beans? After conducting my own research, I asked myself other questions: is decaffeinated coffee safe for consumption? What is the environmental impact of decaffeination methods? In this article, we’ll explore this fascinating process in depth. Discover the different techniques used to remove caffeine while preserving the flavors and distinctive aromas of coffee.
A bit of history
Friedlieb Ferdinand Runge, a German chemist, succeeded in extracting pure caffeine from coffee in 1820. However, he was not seeking to create decaffeinated coffee, but simply to study the molecule. His work therefore did not lead to commercial use.

The first commercial decaffeination process was invented by Ludwig Roselius in 1903 (patented in 1906). The story goes that his batch of beans, accidentally soaked in seawater, had lost a large part of its caffeine while retaining its taste.
As a patron, he supported several artists such as Paula Modersohn-Becker or Bernhard Hoetger. Source Wikipedia
However, his initial method used benzene, now recognized as carcinogenic, and has been abandoned in favor of safer processes.
The shared characteristics of decaffeination methods
In all decaffeination processes, coffee beans are treated before being roasted. The objective is to remove only the caffeine without altering the other aromatic compounds. However, coffee contains hundreds of molecules contributing to its taste, which makes this task particularly complex.

Since caffeine is a water-soluble polar substance, all methods rely on the use of water. But water alone is not selective and also carries away other compounds. This is why agents such as methylene chloride, activated carbon, CO2, or ethyl acetate are used to target caffeine.
The different decaffeination methods
All methods are applied to unroasted coffee beans. After steaming, the beans are rinsed with a solvent or fluid that captures the caffeine. This cycle is repeated 8 to 12 times until a content compliant with standards is achieved:
- 97% less caffeine (American standard)
- 99.9% by mass (European standard)
Methods using organic solvents
The solvents used
The first hazardous solvents have been replaced by dichloromethane and ethyl acetate.
- Dichloromethane is effective and evaporates easily, but it is classified as slightly carcinogenic.
- Ethyl acetate, sometimes derived from sugar cane fermentation, is considered more “natural”.

Interestingly, coffee treated with this solvent is sometimes sold as “naturally decaffeinated”.
Source Maison du Café
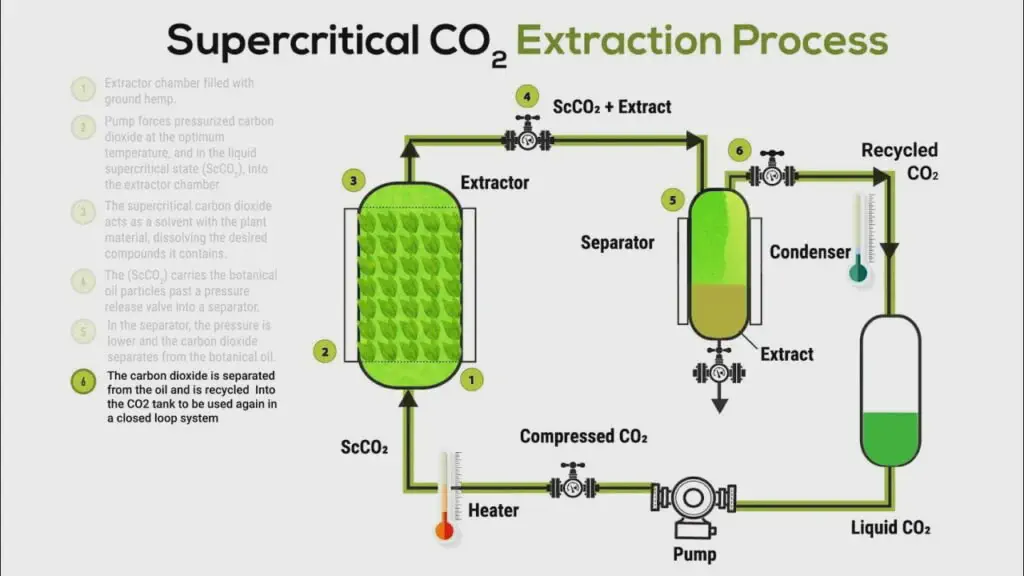
Supercritical CO2 decaffeination
Supercritical CO2 is now a modern and effective method. The beans are placed in a tank where CO2, under high pressure and heat, selectively dissolves the caffeine.
The caffeine-laden CO2 is then released into an absorption chamber, where it becomes gaseous again. The caffeine is filtered and the CO2 recycled.
- Advantage: no harmful chemical solvents.
- Result: good quality decaffeinated coffees, widely commercialized.
Water decaffeination
The gentlest method remains water decaffeination (sometimes called the “Swiss Water” process).
- The beans are soaked in hot water.
- The water is filtered through activated carbon which captures the caffeine.
- The flavor-enriched water is reinjected to preserve the taste profile.
The result is a decaffeinated coffee that respects the aromas and is highly appreciated by enthusiasts.
